Contamination of the Earth’s natural waters is a common malady stemming from human activities. The sources of aquatic and marine contamination are diverse, yet they all ultimately lead to the same final consequences; the death of aquatic species and the inhibition of aquatic habitat resiliency. Oil-spills are arguably the most widely known examples of water contamination, and can severely affect the well-being of aquatic, marine, terrestrial, and avian species. Despite the hazardous effects of oil-spills, the solutions for cleaning them, such as flocculation, oil skimming, and combustion, remain very costly and/or highly inefficient.
Hope is not yet lost however, as there appears to be a new oil-separation technology that most might not predict. Foam! Yes, foam, that light-as-a-feather-stiff-as-a-board material we all know and love. However, the foam that is the subject of this post is no ordinary insulating or packaging foam. The foam I speak of is developed through several chemical processes that ensure it is both superhydrophobic (water rejecting) and superoleophilic (oil accepting), which is a crucial property in water-separating materials.
First, the surface of a polyurethane foam sheet is coated with a graphite colloid, on which copper is then electroplated. The foam sheet is then placed into an aqueous solution of sodium hydroxide and potassium persulfate. The reaction between the copper and the aqueous solution results in the formation of complex nanostructures of copper hydroxide (Cu(OH)2) on the foam surface.
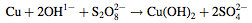
The foam is then treated with n-dodecanethiol dissolved in ethanol, which reacts with the copper hydroxide to form an organic copper compound (Cu(SC12H25)2).
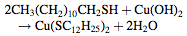
The formation of the organic copper compound is important for three reasons: (1) the organic copper compound replaces the metal oxide which prevents the metal oxide from reacting and degrading from exposure to water, (2) the formation of an organic surface substrate allows for other organic substances to pass into the foam very easily, and (3) the organic copper compound retains the nanostructure network formed by the copper hydroxide.
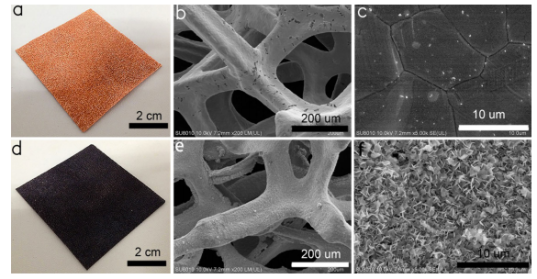
The third reason mentioned above is the most important in ensuring that the copper foam does not allow water past the surface. Nano structures such as these form a rough surface for water molecules. This rough surface does not allow water to pass through it because water has very strong cohesive properties, meaning that it would rather stay as a droplet than form smaller individual micro-droplets that can pass through the pore size of the foam. Additionally, once the foam is wetted with oil, the water will simply not pass because oil and water do not mix.
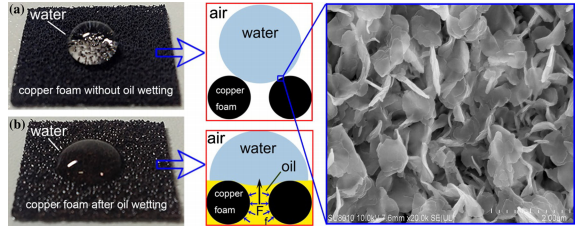
All in all, treated copper foam presents tremendous possibilities for more efficient oil-spill cleanup, yet extensive field testing is still required to determine the practicality of superhydrophobic/superoleophilic foams. I for one have concerns relating to large scale (coastal/oceanic-scale) implementation of treated copper foam and similar materials. However, I am optimistic that such materials could be effectively used in smaller scale cleanup operations, where the actual volume of oil in water is limited to more manageable quantities. Additionally, the economic, energetic, and environmental feasibility associated with producing the metallic foam is uncertain. Foam is relatively cheap, but what about the energy demands of electroplating or the toxicity of the waste produced from creating the chemicals required to treat the copper foam? What are some thoughts you have? Please share any comments, questions, or concerns below.
References:
Zhang, J., Ji, K., Chen, J., Ding, Y., & Dai, Z. (2015). A three-dimensional porous metal foam with selective-wettability for oil–water separation. Journal of Materials Science, 50(16), 5371-5377. doi:10.1007/s10853-015-9057-2
This is a very interesting solution to the problem, which I guess is sprayed as a foam? As to the feasibility of the solution, copper is not exactly free, but it is a relatively cheap metal. The electricity used in the electroplating would be the kicker for cash, it seems. Depending on the efficiency of the treatment, and the quantities produced with each batch, the price to produce could be worthwhile. It may produce toxic waste when produced, but as with gas emissions, a point source is much more preferred to wide-spread pollution because the problem, however nasty, can be handled in one place that is known. I think the efficiency of the treatment, and whether cheap electricity is available would be the two defining questions of the feasibility of the undertaking.
LikeLiked by 1 person
Like Tyler touches on, even though the point source is still not necessarily a rosy environmental option, it still may prove to be a better option due to our ability to better control the environmental drawbacks. Another issue that field testing may come to tackle with this material is what they do with the used foam. Ideally one would hope they can chemically recover the oil from the foam and then use it, but I wonder what other products such a chemical reaction may produce – as in will the method they find that can remove the oil from the foam not also create a secondary toxin that we may have to worry about. Another issue may be how we dispose of the used foam. Creating a new type of hazardous waste may open up doors to new issues of disposal, but the potential to clean up oil spills can certainly outweigh these costs!
LikeLike
I am a little confused about the application of this in an oil spill scenario. In an oil-skimming process, wouldn’t you want a material to catch oil and reject water, not the other way around? I can definitely see its use as a filtration device for a contaminated tank of water or something similar though. Just pouring it over the treated copper foam can easily separate the oil from water. I also wonder how long the foam would last, how many treatments it can do, or if it is recyclable. I do think it is a great piece of technology that can have a wide number of industry and environmental applications.
LikeLike
This was an interesting read. I had no idea that this solution even existed. How long/often has this method been used? Are there any known/reported negative impacts associated with this besides waste produced with the copper foam? Overall, I do think this is an excellent solution to oil spills and saving aquatic life.
LikeLike
What a great idea! I am interested in how feasible it actually is though. What resources are used in industry to create the foam? Can it be made, at least in part, with recycled materials? Can it be easily recycled once it has absorbed an amount of oil? Also, if you “squeeze” the oil out of the foam after it has soaked up oil can the oil still be used? If it can not be recycled once it has been used with oil, what are the appropriate disposal methods? Would we be solving one problem with another? This is a great topic and I will definitely look into it further!
LikeLike
The foam itself is cheap but how expensive is the process to plate it and how expensive is the disposal of the product. Would they just burn the foam after use? Maybe there is a method to extract the oil from the foam and still use it. Oil spills are a large aquatic environmental issue that will reduce as we reduce the amount of off shore drilling. Do you think the demand for a product like this will decrease as more renewable energy sources begin to be implemented?
LikeLike
I think it all boils down to the number of dollar signs attached to such a process. Yes it may work, but at what cost? It would be a shame if it were more expensive to clean up the oil spill with this foam material versus just paying the sanctioned fines like BP had to pay. Also the problem is designing a method of delivering the foam to scale. Yes it may work in a lab, but will it work when you need thousands of square miles of this stuff to clean up an oil spill. Its an interesting read, and it shows that people are researching alternative methods of managing oil spills. I hope this research can be used to help develop a clean up method that is cheap, quick to install, and effective in managing oil spills. Interesting read!
LikeLike
I think what it all boils down to is price. How much will it cost and who will foot the bill? I agree that this would only be viable on small scale operations because with the size of the ocean, the currents and tides carry the oil all over the place. Also, Im not sure on how big of a market there would be for this technology. I don’t know how many oil spills occur each year. The only two one I know of were the recent BP one and another awhile ago off the coast of California. So is this something that we need to be putting a ton of money into if it doesn’t happen all that often? I do like the concept, but I don’t think the oil spills are frequent enough to warrant the money for research.
LikeLike
My question is if this is introduced on a wide scale (i.e. BP Oil Spill), then how could crews then clean up the foam? Just like buoys or absorbent materials already dispatched, the larger issue then becomes how to clean up the foam? It seems like a much more difficult operation when you start to consider the foam is probably harder to retrieve than buoys. Just something to think about…
LikeLiked by 1 person
Awesome read! My first thought was similar to Grant’s- what about the clean up? But if the foam can get the job done then it is definitely worth it. At the least, maybe oil spill foam could create jobs by needing a large staff on hand to retrieve the saturated foam?
LikeLike
I like how we are finding new solutions to the problem we created. We often have so many negative impacts on the environment and they have detrimental effects to the ecosystem. When BP had it’s infamous oil spill it affected so many things. I do wonder the affect it could have on such a massive oil spill such as BP though. It seems near impossible with how vast an oil spill can be which would probably spread throughout the ocean.
LikeLike
I’m picturing pieces of foam board floating in the ocean, so I suppose they could be contained with some sort of net system? That being said, I agree with everyone else in wondering what the environmental impact of this would be. Could the copper compounds detach from the foam and enter the aquatic system? And again, what do we do with it when it’s been used? If there’s a way to get the oil off of the copper maybe refineries would like this technology as a way to reduce their losses to spills and leakage.
LikeLike
Interesting article, I hadn’t heard of this before! A couple people have mentioned similar concerns about clean up – but just to ask the obvious, this stuff floats right? Even after its weighted down with oil and organic material? I’m assuming yes since if it flocculates and sinks it’d be extremely difficult to clean up, as well as extremely difficult to field test the long term effects on the ecosystem. I think you’re right that this kind of thing probably has more applications in small scale clean ups than major oceanic oil spills. It’d be very difficult to predict the impact of dropping large quantities of this stuff on a complex aquatic ecosystem, but this technology potentially could have some neat industrial/stormwater/urban applications.
LikeLike
It amazes me the innovation of people for something seemingly so simple but also complex. My biggest concern would be heat release back into the water which could create damaging effects just as chemical contaminants do. Not sure if they’ve looked at heat changes but definitely something for experimentation.
LikeLike
ONe symptom this method could introduce is leeching from the foam into the water. I am not too sure of the leaching habits of polyurethane based foams. But with the introduction of direct sunlight on the water as well as the abrasive qualities of saltwater, I imagine the leaching rate would be exacerbated. While this leaving may not be as much of a threat as the problem it is being used to remedy. , it could be an unintentional introduction
LikeLike
I was not aware of this technology and I found this article to be very interesting. The applications this can be used for will be endless. This could revolutionize water treatment when it comes to oil spills, which in today’s world seems to be often. As for the detrimental effects on the environment, we will just have to see the results of the testing you mentioned are being performed. Hopefully, if all goes well, this will be implemented in the near future and make the cleaning of oil spills a lot more effective.
LikeLike
This is really interesting. I am a little bit concerned about the possible pollution that could be produced from creating the foam; however, I believe that in this case, the benefits outweigh the consequences. I would much rather we create more pollution if it meant we could save our oceans. Oil spills can be devastating to an ecosystem and I think the faster and more efficient we get in cleaning up the oil, the better.
LikeLike
This foam seems like a necessary innovation to me. If the foam is very effective at removing oil from water, then this could solve a lot of problems in our world of contamination. The problem is that this is a after the fact kind of solution as opposed to a preventative solution. While this will be helpful to mitigate the damage already caused, we will still need to work on making it harder for these oil spills to occur in the first place.
LikeLike
This seems like a great idea, but I think it will need a lot more thought put into it before it can be implemented effectively on a larger scale. I , like other commenters, wonder what the process is once the foam has been applied. One person mentioned that they were wondering if you could squeeze the oil out of the foam and reuse it. It seems highly unlikely but it would be really cool to be able to do that, use the absorbed oil for something useful, and then reuse the foam as well. Like i said, it sounds kinda far fetched but who knows…
LikeLike
This seems like an extremely viable option for oil-spill recovery, however I am wondering about the disposal/reuse of the foam. After the foam becomes saturated with oil how does the process proceed? Is the foam (with oil) disposed of as is, or can the oil be removed in order for the foam to be reused?
LikeLike
Has it been implemented in the real world? I think it is too early to say whether it is effective
LikeLike
This is a really neat idea. I agree that this seems like more of a smaller scale solution to oil spills. I don’t think this process could be implemented on a large enough scale in the ocean to be efficient yet. I also think the end of life process for the oil once it is absorbed needs to be elaborated on. Where will the oil go? What is the lifespan of the foam? I’m also very curious to the cost of this process as well.
LikeLike
I’m curious, do you know who initiated these efforts? Is it oil companies or outside efforts?
LikeLike
This is a neat idea. Never heard of this technology before. I’m curious though as to how they would clean up the foam after it has done its job. Also, what would they then do with the foam after it has collected? Is this a solid foam or a foam that can be sprayed? What would happen to any foam that wasn’t recaptured? It certainly is an interesting idea and I’m curious as to how this will affect the future.
LikeLike